This seemingly innocuous question has sparked curiosity and debate among oenophiles and science enthusiasts alike. While the idea of setting fire to a glass of Merlot may seem like an odd experiment, the potential flammability of wine raises intriguing questions about its chemical composition and historical significance.
Key takeaways
- wine is not flammable due to the low alcohol content of up to 15 % ABV
- even when heating up wine the vapours are not flammable due to the high water content of the fumes
- brandy or spirits with an alcohol content higher than 50 % ABV are flammable
Is Wine Flammable?
From ancient rituals involving fiery libations to modern-day concerns about alcohol safety, exploring whether wine can ignite offers a fascinating glimpse into the intersection of tradition, science, and practicality.

Non fortified wine is not flammable even when using to cook it due to the low alcohol content. Only fortified wines and spirits with an ABV higher 50 % are flammable.
Exploring the Flammability of Wine
Exploring the flammability of wine is an intriguing journey that delves into the alcohol content and its potential to ignite. While most people associate flammability with high-proof spirits like vodka or whiskey, it’s surprising to discover that even a seemingly innocuous glass of wine can also catch fire under the right conditions. This phenomenon is due to the alcohol content present in wine, typically ranging from 9% to 16%. When exposed to a flame, this alcoholic concentration becomes volatile and can ignite, resulting in an unexpected display of pyrotechnics.

What is the minimum percentage of alcohol to make it flammable?
When it comes to setting alcohol on fire, the exact percentage needed can vary depending on the type of alcohol. In general, most spirits with a high proof (over 50% alcohol by volume) are flammable. This includes beverages like overproof rum, grain alcohol, and high-proof vodka. However, lower-proof beverages like beer and wine do not contain enough alcohol to sustain combustion.
It’s important to note that high-proof alcohols can be lit on fire. Doing so can be extremely dangerous and should not be attempted casually. The flames produced when burning alcohol are nearly invisible and can quickly escalate out of control. Additionally, the fumes from burning alcohol can also present a serious risk if inhaled.
In conclusion, the percentage of alcohol needed for it to burn largely depends on the proof of the beverage. Higher percentages of alcohol make a substance more likely to ignite. It’s crucial to emphasize that attempting to light alcoholic beverages should be done with extreme caution and only under appropriate supervision.
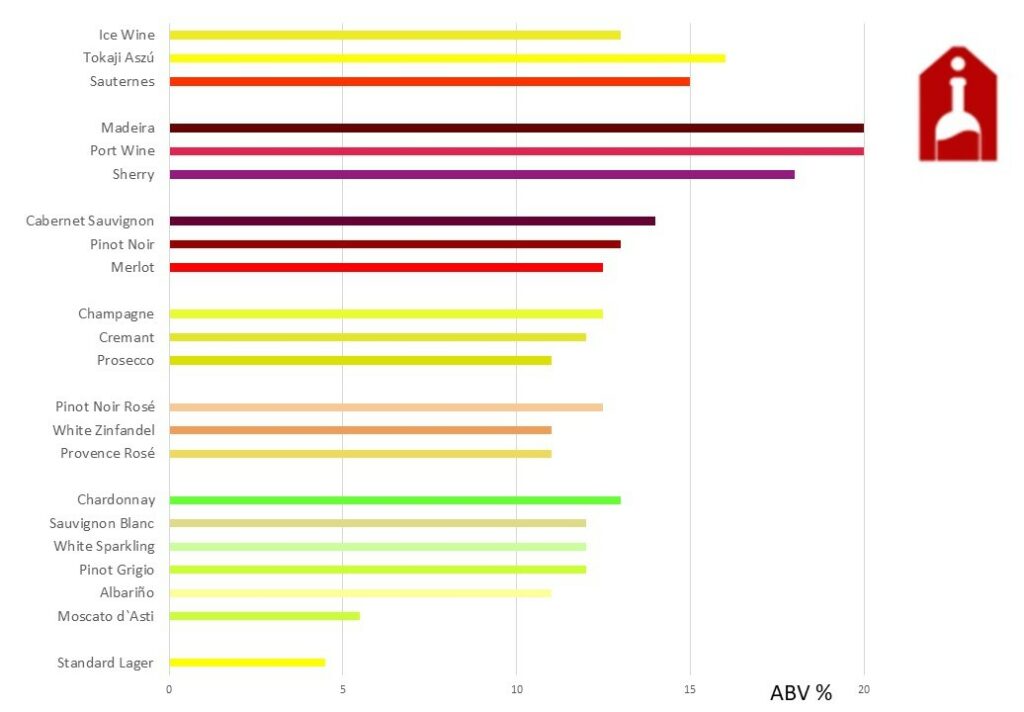
The Alcohol Content in Wine
When it comes to the alcohol content in wine, there is a wide spectrum to explore. From the lowest content found in some sweet white wines, typically around 5-7%, to the highest levels in fortified wines like Port or Sherry, which can reach up to 20% or more. It’s fascinating to consider how these varying alcohol levels can significantly impact the flavor profile and body of a wine. The lower alcohol wines may have a lighter, more refreshing quality. Those with higher levels often exhibit richer, more intense characteristics.

Additionally, it’s interesting to note that factors such as grape ripeness, climate conditions, and winemaking techniques all contribute to the final alcohol content of a wine. For instance, warmer climates tend to produce grapes with higher sugar levels. It translates into higher alcohol in the finished wine. Understanding these nuances can enhance one’s appreciation for different wine styles and regions. Whether you prefer a light-bodied Moscato or a robust Zinfandel, recognizing how alcohol content plays a role adds another layer of complexity and enjoyment to the world of wine.
Flash Point and Ignition Temperature
Understanding the flash point and ignition temperature of ethanol-water mixtures is essential for safely handling and transporting these solutions. The flash point of a liquid is the temperature at which it gives off vapor that can ignite when exposed to an open flame or spark. When water and ethanol are mixed, this not only affects their flash points but also changes their behavior in terms of flammability. Interestingly, as the proportion of water in the mixture increases, the flash point also increases due to water’s higher boiling point compared to ethanol.

However, it’s crucial to note that even high-water content mixtures like vodka can still catch fire if subjected to extreme heat or an open flame. This distinction highlights the importance of understanding both the flash point and ignition temperature when working with ethanol-water mixtures. Moreover, these insights underscore the need for proper storage and handling practices. They help to prevent accidents involving flammable liquids such as ethanol-water solutions.
Conclusion: Understanding the Flammability of Wine
One can attribute the non-flammability of wine to its relatively low alcohol content and high water content. This makes it less susceptible to catching fire. Understanding this property of wine is important for ensuring safety in various settings, such as restaurants, wine storage facilities, and transportation. Additionally, knowing the non-flammable nature of wine can help dispel misconceptions and prevent unnecessary panic or alarm in emergency situations. It is crucial for individuals working with or around wine to be aware of its non-flammable properties and handle it responsibly. Ultimately, by increasing awareness and knowledge about the non-flammability of wine, we can promote a safer and more informed approach to its use and handling.

